
Background: Hypomethylating agents (HMA) form the current standard treatment for patients with higher-risk myelodysplastic syndrome (HR-MDS) who are not eligible for allogeneic hematopoietic stem cell transplantation (HSCT). However, overall response rates (ORRs) remain low in patients receiving azacitidine (Aza), and median overall survival (OS) is reported as ~15 months (Sekeres et al. J Clin Oncol. 2017). In addition, there are few data on patient-reported outcomes (PROs) published in this population while on treatment. Venetoclax (Ven) is a selective, potent, orally bioavailable BCL-2 inhibitor, which has demonstrated synergy with HMA in preclinical studies of HR-MDS. From an ongoing, open-label, dose-escalation, Phase 1b study (NCT02942290) evaluating Ven+Aza for the treatment of treatment-naïve HR-MDS, we report the updated safety and efficacy in all treated patients and the exploratory analysis of key PROs in patients who received the recommended Phase 2 dose (RP2D).
Methods: Patients aged ≥18 years with treatment-naïve HR-MDS, International Prognostic Scoring System intermediate-2 or high, bone marrow blasts <20% at baseline, and an Eastern Cooperative Oncology Group (ECOG) score ≤2 were enrolled; patients with chronic myelomonocytic leukemia or therapy-related MDS and candidates for intensive chemotherapy or HSCT were excluded. Ven was initially given at a dose of 400 mg or 800 mg for 28 days in a 28-day cycle. Due to intolerance among patients with MDS, this was later amended to an escalating dose (100, 200, and 400 mg) for 14 days in a 28-day cycle. Aza was administered at 75 mg/m2 subcutaneously or intravenously on Days 1-7 of each 28-day cycle. The primary objectives of the study were to assess the Ven+Aza safety profile and to establish the RP2D. Key secondary objectives included assessment of ORR and OS. Safety and efficacy assessments were carried out on all patients who received ≥1 dose of study drug, and efficacy endpoints were evaluated according to the 2006 International Working Group response criteria, with OS analyzed using Kaplan-Meier methodology. PROs were exploratory and included the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core (EORTC QLQ-C30) scale.
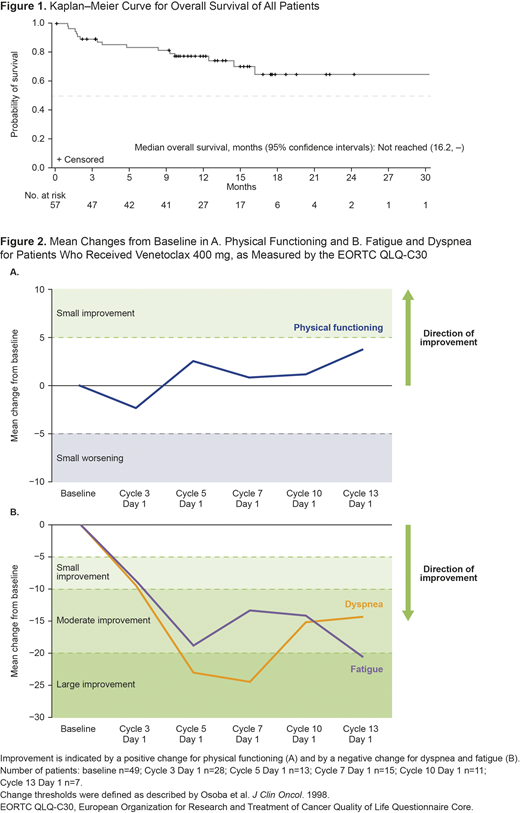
Results: At data cutoff, December 31, 2019, 57 patients had received Ven+Aza, with a median follow-up of 13.0 months (95% confidence interval [CI] 11.3, 15.6 months). The majority of patients were male (75%); median age was 71 years (range 26-85 years); and 89% had ECOG score 0-1. All patients experienced ≥1 adverse event (AE), the most common being constipation (54%), neutropenia (51%), and nausea (51%). Grade ≥3 AEs were experienced by 97% of patients, with neutropenia (51%), febrile neutropenia (46%), and thrombocytopenia (30%) the most common. Febrile neutropenia was the most common serious AE (42%). The 30-day mortality rate was 2%. The ORR was 77%, including complete remission (CR) and marrow CR (mCR) achieved by 42% and 35% of patients (of whom 40% achieved mCR + hematological improvement), respectively; none achieved partial remission. Median OS was not reached (95% CI 16.2 months, not estimable; Figure 1). Median duration of response was 14.8 months (95% CI 12.9 months, not estimable). Median progression-free survival was 17.5 months (14.5, not estimable). Of the patients who received the RP2D of Ven 400 mg for 14 days/28-day cycle in combination with Aza, physical functioning, as measured by the EORTC QLQ-C30, was maintained through 48 weeks of treatment. In addition, clinically meaningful improvement in fatigue and dyspnea, as measured by the EORTC QLQ-C30, was achieved by the beginning of Cycle 5 and was maintained through Week 48 (Cycle 13; Figure 2).
Conclusions: The combination of Ven+Aza demonstrates promising efficacy, including response durability, and an acceptable safety profile for patients with HR-MDS. Maintenance in physical functioning and clinically meaningful improvement in dyspnea and fatigue were observed throughout the first 48 weeks, although these data are not yet mature and low patient numbers beyond Cycle 7 limit conclusions. Additional follow-up data and correlation with disease risk features including mutations will be presented at the meeting.
Garcia:Pfizer: Research Funding; Eli Lily: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wei:Amgen: Consultancy, Honoraria, Research Funding; MacroGenics: Consultancy, Honoraria; Servier: Consultancy, Honoraria, Research Funding; Walter and Eliza Hall Institute: Other: former employee and receives a fraction of its royalty stream related to venetoclax; Pfizer: Honoraria; Genentech: Honoraria; Astra Zeneca: Honoraria, Research Funding; AbbVie Inc.: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding. Borate:Genentech: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Research Funding; AbbVie: Other: Investigator in AbbVie-funded clinical trials; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Fong:Astellas: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; AbbVie: Honoraria. Baer:Forma: Other: Institutional research funding; Astellas: Other: Institutional research funding; AbbVie: Other: Institutional research funding; Incyte: Other: Institutional research funding; Kite: Other: Institutional research funding; Oscotec: Other: Institutional research funding; Takeda: Other: Institutional research funding. Nolte:AbbVie: Other: Investigator on an AbbVie funded clinical trial. Jurcic:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Arog Pharmaceuticals: Research Funding; Astellas: Research Funding; Forma Therapeutics: Research Funding; Genentech: Research Funding; Kura Oncology: Research Funding; PTC Therapeutics: Research Funding; Syros Pharmaceuticals: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jacoby:Takeda: Consultancy; AbbVie: Research Funding; Jazz Pharmaceuticals: Research Funding. Hong:F. Hoffmann-La Roche: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Platzbecker:Geron: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Amgen: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Odenike:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, NS Pharma, Gilead Sciences, Janssen Oncology, Oncotherapy, Agios, CTI/Baxalta, Aprea: Other: Institutional research funding; Astra Zeneca: Research Funding; Incyte: Other: Institutional research funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Impact Biomedicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cunningham:AbbVie: Research Funding; Amgen: Research Funding; Astex: Research Funding; Celgene: Research Funding; Janssen: Research Funding; Novartis: Research Funding; Principia Biopharma: Research Funding; Rigel Ilona: Research Funding. Zhou:AbbVie: Current Employment, Other: may hold stock or other options. Tong:AbbVie, Inc.: Current Employment, Other: may hold stock or other options. Hogdal:AbbVie: Current Employment, Other: may hold stock or other options. Kamalakar:AbbVie: Current Employment, Other: may hold stock or other options. Hutti:AbbVie Inc.: Current Employment, Other: may hold stock or stock options. Kye:AbbVie: Current Employment, Other: may hold stock or other options. Garcia-Manero:Novartis: Research Funding; Merck: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Amphivena Therapeutics: Research Funding; AbbVie: Honoraria, Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; H3 Biomedicine: Research Funding; Jazz Pharmaceuticals: Consultancy; Acceleron Pharmaceuticals: Consultancy, Honoraria; Onconova: Research Funding.
Venetoclax is a BCL-2 inhibitor approved for use in CLL and in combination with azacitidine, decitabine, or low-dose cytarabine for the treatment of newly-diagnosed AML who are 75 years or older or who have comorbidities that preclude use of intensive induction chemotherapy; the current clinical trial reports use in MDS
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal